Recently, researchers at Shanghai Jiao Tong University, led by Investigator Tao Fei, collaborated with scientists from the Massachusetts Institute of Technology in the United States to apply a simple method known as the "QTY code" to the transmembrane receptor histidine kinase CpxA, completely transforming it into a water-soluble form.
The engineered CpxA not only exhibits the expected biophysical properties but also retains its inherent natural molecular functions to a high degree, including autokinase activity, phosphotransferase activity, phosphatase activity, and signal receptor activity involving the water-soluble transmembrane domain, among others.
Additionally, the research team explored the principles of structural stability and activity balance within the water-soluble transmembrane domain and found that a dense and dynamic hydrogen bond network introduced by the QTY code may play a key role.
As this achievement marks the first time a water-soluble membrane protein with complete functionality has been realized, it not only provides guidance for the future design of water solubility and functionality of membrane proteins but also holds the potential for broad applications in the fields of synthetic biology, drug discovery, biosensing, structural biology, and beyond.
Advertisement
For example:Firstly, in the field of drug discovery, histidine kinase proteins can be targeted to develop more antibiotic products, thereby achieving control over microorganisms through different mechanisms of action.
Secondly, in metabolic engineering, the metabolic network of cells can be effectively manipulated by modifying or introducing specific histidine kinase proteins.
Thirdly, in the field of biosensing, sensors based on histidine kinase proteins can be developed for the detection of small molecules, odors, and other substances.
QTY Code: A feasible method for designing water-soluble membrane proteins.Membrane proteins refer to proteins that exist in biological membranes such as cell membranes and organelle membranes, accounting for about 20% to 30% of cellular proteins, and they play an important role in various life activities. For example, they maintain the stability of cell structure, conduct material transport, and are responsible for signal transduction.
Therefore, studying them is a crucial part of exploring biological processes and is also at the core of life sciences and modern medicine.
However, due to their highly hydrophobic nature, studying membrane proteins is extremely challenging, facing various challenges such as high costs, low expression levels, labor-intensive detergent screening, and difficulty in obtaining high-quality crystals.
Thus, as of November 2021, the proportion of membrane proteins among all proteins that have had their structures resolved is less than 1%[1].
Redesigning the membrane-bound regions that contain a large number of hydrophobic amino acids into water-soluble forms is a feasible solution to the aforementioned problems.Currently, numerous studies have demonstrated that water-soluble variants designed based on this scheme can exhibit varying degrees of solubility while maintaining certain structural characteristics.
However, it is regrettable that these studies can only help maintain limited functions of membrane proteins, and cannot fully preserve their intrinsic molecular functions.
To overcome the aforementioned limitations, researchers in this field need to balance at least the following three aspects:
Firstly, solubility. This allows the protein to remain stable in a lipid-free environment.
Secondly, stability. This is used to prevent the collapse of the originally correctly folded structure when a large number of mutations are systematically introduced.Once again, activity. This can prevent the destruction of the interaction network that is very important for intrinsic biological activity.
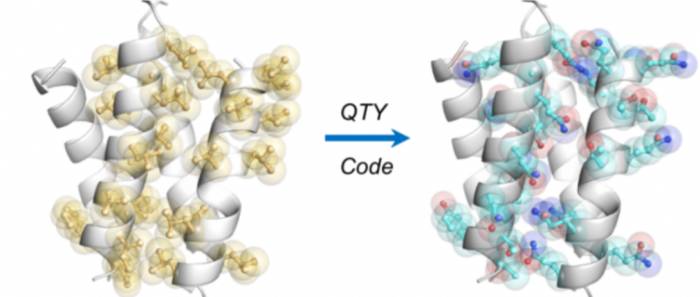
From 2011 to 2018, a collaborative team from Shanghai Jiao Tong University and the Massachusetts Institute of Technology spent 7 years inventing the QTY code for protein engineering modification, especially for the hydrophilic modification of membrane proteins. (Editor's note: In the QTY code, Q represents glutamine (Gln, Glutamine), T represents threonine (Thr, Threonine), and Y represents tyrosine (Tyr, Tyrosine).)
This method aims to make proteins that are not originally soluble in water become water-soluble, in order to promote biochemical research while maintaining their natural conformation and biological function.
Compared with other methods, the QTY code has two major advantages: one is that it is simple and easy to operate, without relying on complex computer programs; the second is that it can directly design the sequence without the need to input existing structural data.
It is understood that in the past few years, this method has been successfully applied to the design of water-soluble membrane proteins, and has demonstrated good water solubility, stability, and affinity for ligand binding.However, the extent to which it can help retain the biological activity of membrane proteins, especially the catalytic activity of enzymes, still requires further research.
Using the QTY code for histidine kinases to achieve water-soluble transmembrane receptor proteins with biological activity and complete molecular functions
Based on this, in this study, the team attempted to apply the QTY code method to the transmembrane receptor object of histidine kinases.
Not only did they achieve the water solubilization of membrane proteins with biological activity for the first time, but they also retained the expected biophysical properties and the complete functional maintenance of the transmembrane structural domains of the histidine kinase protein."The transmembrane region is responsible for signal transduction. Our research allows histidine kinase proteins to undergo signal transduction and maintain their original conformational changes even after being completely transformed into water-soluble forms," explained Li Mengke, a doctoral candidate at Shanghai Jiao Tong University's Zhiyuan Honors Program.
So, what kind of methodological advantages have enabled researchers to preserve the biological activity of membrane proteins?
In fact, from the principle of water-solubilization design, the basic goal can be achieved by simply replacing the hydrophobic amino acids on the surface of the protein with hydrophilic ones.
"But the matter itself is not that simple, because even after replacing the amino acids, it will still have a significant impact on the entire protein structure. Therefore, the main innovation of our work is that while changing the hydrophobicity of the transmembrane region, we also optimized the interaction network that stabilizes the protein structure," said Tao Fei.
Additionally, it is equally intriguing as to why the research group chose to study histidine kinase, a specific type of membrane protein, as the subject of their research?In response to this, Tao Fei explained: "This is because histidine kinase is very important for microorganisms. To put it in perspective, if we consider a microbial cell as a person, histidine kinase is akin to the person's eyes, nose, or ears, capable of recognizing the environment and sensing."
Therefore, the team's research on it helps to lay the groundwork for more future work related to microorganisms, such as the engineering transformation of cyanobacteria, a chassis for negative carbon synthetic biology.
That is to say, on one hand, this research provides a universal method for studying microbial sensory proteins, so researchers in the field, including the team, can base their subsequent similar protein research on this method, first solubilizing the target protein in water before proceeding with the desired research.
On the other hand, the improved QTY code can not only be applied to the solubilization of membrane proteins but also provide a reference for studying other membrane proteins.Validate the hydrogen bond network introduced by the QTY password, which plays a key role in the transmembrane signal transduction of the CpxA protein.
As mentioned earlier, after the team found that the designed histidine kinase CpxA had good performance consistent with the wild-type protein in terms of molecular function and biophysical properties, they became very interested in the mechanism behind it.
Therefore, they tried to explore the balance between the solubility, stability, and activity of the water-soluble transmembrane domain, trying to find the reason why the histidine kinase CpxA maintains stability and functions.
Specifically, they first used the AI tool AlphaFold2 to predict the structure of the CpxA protein, and then analyzed the interaction network in the protein through molecular dynamics simulation.
Li Mengke said: "We found that there is a relatively dense and dynamic hydrogen bond network, which can not only maintain the stability of the protein but also mediate the conformational change process required for its signal transduction."To verify that the hydrogen bond network plays a key role in the transmembrane signal transduction of the CpxA protein, researchers performed mutations on some sites that are crucial to this network in molecular dynamics simulations, and then found that the protein immediately lost its signal transduction function.
"This indicates that our hypothesis is essentially correct," said Li Mengke.
According to him, the research took more than four years. During the process, they encountered various difficulties.
For example, once when Li Mengke thought that the prepared sample protein might have failed, he threw it away.
But later, he carefully re-analyzed the gel filtration chromatogram and found that the original understanding was problematic, and the protein might be functional. He picked it up from the trash can and retested it the next day, eventually obtaining the desired results.In my view, this is not only an interesting little story, but also an important turning point in our research, said Li Mengke.
Recently, the relevant paper was published in Nature Communications under the title "Design of a water-soluble transmembrane receptor kinase with intact molecular function by QTY code" [2].
The paper's reviewers commented, "If the QTY code can be applied so successfully, it will greatly promote the development of the field."
In addition, it is understood that Tao Fei's research group is currently mainly focused on research in the field of microbial synthetic biology, and the carbon-negative chassis organism cyanobacteria is one of the main chassis of the research group.
Therefore, he also plans to lead the team to carry out more histidine kinase protein research related to cyanobacteria, hoping to clarify their specific functions while developing them as targets to control the metabolism of cyanobacteria.At the same time, he also looks forward to seeking some collaborators interested in drug development, targeting histidine kinase proteins to jointly develop some antibiotic products.
